Join the first multicenter Real-World study for Lumbar Disc Decompression with QMR®
Be part of a real clinical data revolution with technology as RESADISC®
Over 30 centers
Innovative QMR® technology
One shared goal: reduce invasive surgery
The Purpose Behind This Study
Chronic lumbar radiculopathy affects millions, and most clinical data is lost. QMR® is an innovative, minimally invasive solution — but needs real evidence to support large-scale adoption.
Real World Evidence
Clinical data captured in real-time from hospital practice.
QMR® Characterization
Controlled, targeted, histologically verified disc decompression.
Artificial Intelligence
Smart platform for outcome prediction and data security.
Scientific Impact
Publication in high-impact journals and improved clinical algorithms.
About QMR® Disc Decompression
QMR® (Quantum Molecular Resonance) is an advanced coablative radiofrequency technology that allows targeted disc decompression while preserving surrounding tissue. Combined with percutaneous microdiscectomy, it offers a new approach to treat chronic lumbar radiculopathy without major surgery. Unlike traditional surgery, QMR® is:
Controlled low
temperature
Focused within 1mm of the affected disc
Proven histologically to preserve healthy structures
Minimally
invasive
Dr. Raquel Peláez – Principal Investigator of the RWE Study
This is the text area for this paragraph. To change it, simply click and start typing. Once you've added your content, you can customize its design by using different colors, fonts, font sizes and bullets. Just highlight the words you want to design and choose from the various options in the text editing bar.
Why this study?
Despite promising results, most clinical data on QMR® disc decompression is limited or fragmented. Real-world clinical evidence (RWE) is urgently needed to:
1
Confirm the safety and effectiveness of this technique in daily hospital practice
2
Generate high-impact scientific publications
3
Improve treatment algorithms for chronic back pain
4
Short heading goes here
How your participation works
- No change in patient care routines
- Register each case in less than 5 minutes
- Secure digital platform
- Scientific visibility and co-authorship
- Kick-off: September 2025
Apply as Investigating Center
The clinical kickoff starts September 2025
Founding centers will lead international recruitment, gain early co-authorship,
and become reference sites for global scientific leadership.
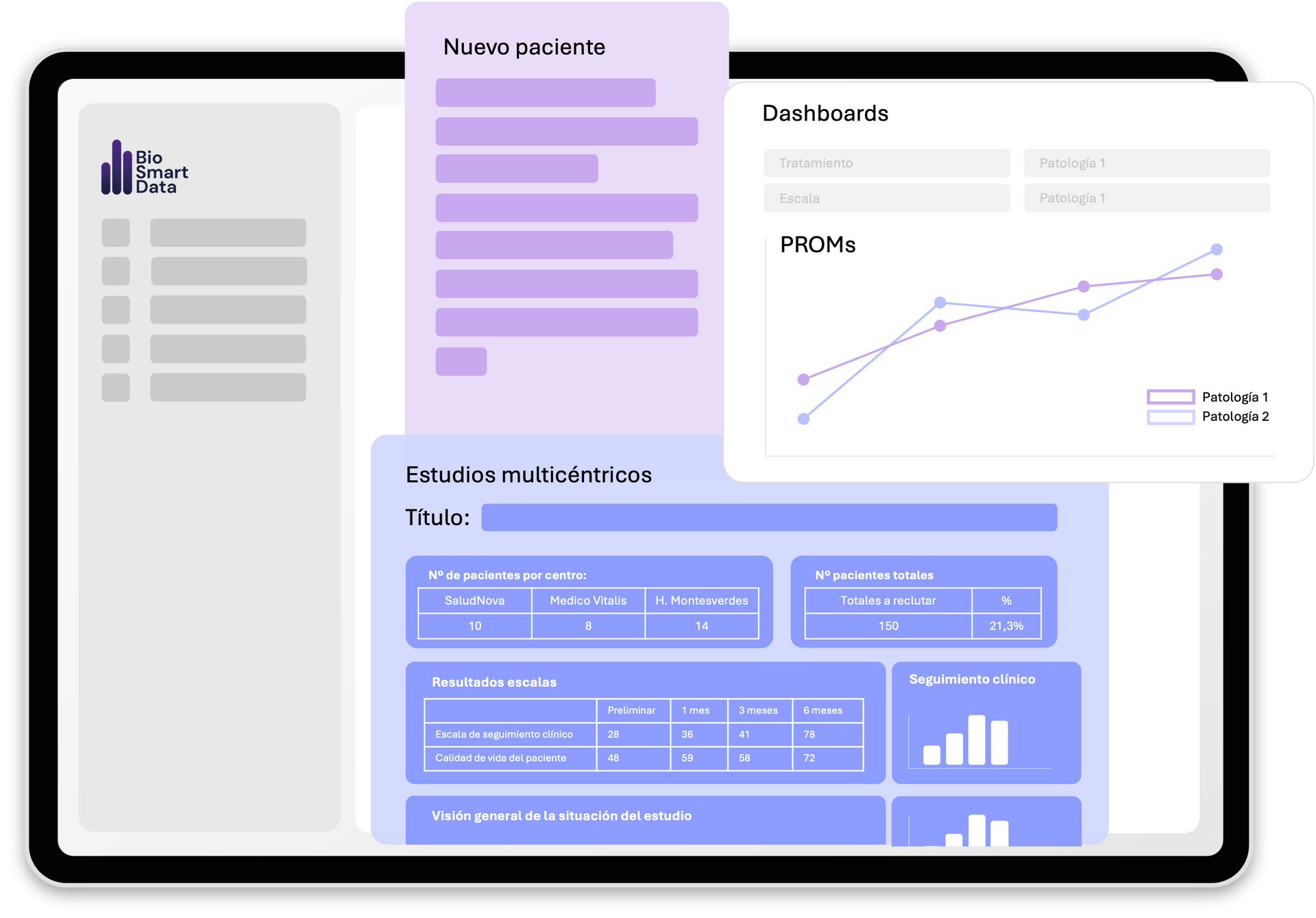
THE TECHNOLOGY BEHIND
Powered by BioSmartData Technology
- Fully digital eCRD
- GDPR/ENS-compliant
- AI-assisted secure analytics
- Easy onboarding process
FAQs
Who can participate?
Any clinical center, hospital or private practice with experience in disc decompression. Both public and private institutions are welcome.
What is the cost to participate?
Participation in the study itself is free of charge. Centers only assume the costs associated with acquiring the technology required to operate:
• The BioSmartData® software license
What technology do I need to participate?
• A valid BioSmartData® Software license (cloud-based, easy installation).
• Internet connection to access the platform securely.
How long is the follow-up period for each patient?
Each patient will be monitored for a minimum of 12 months, allowing us to collect both short- and mid-term clinical outcomes.
What type of data is collected?
The platform captures structured clinical data directly from your routine practice.
Who owns the data?
All data is fully anonymized and managed under strict ethical standards. The study database is a collective scientific repository governed by the international research consortium and scientific committee.
Each participating center maintains access to its own cases, and global aggregated data will be used for joint scientific publications.
How is patient data protected?
Patient privacy and data security are top priorities. The platform ensures:
• Full data anonymization
• AES-256 encryption protocols
• GDPR compliance (Europe)
• ENS High Level Security (Spain)
• Controlled access only for authorized research staff







